- The Science Content Lab
- Posts
- Competitive Intelligence Meets Science Storytelling
Competitive Intelligence Meets Science Storytelling
How Biotech & Medtech Brands Build Credibility Through Market Awareness

Welcome, reader!

SciRio crosses a milestone: A Personal Note from the Founder
SciRio is now a Private Limited company. Five years ago, I left my lab at IISc to pursue something most people couldn't understand—making science accessible through storytelling. It felt reckless then. It still feels audacious now. We've come a long way from those early pandemic days when I was a solo founder trying to convince companies that science communication wasn't optional, it was essential. Today, we're a team. We're working with incredible organizations in healthcare, biotech, and life sciences. We're building something that didn't exist before—a space where scientific rigor meets compelling narrative. This milestone means we're ready to scale. We're looking ahead to new markets, new ways of working, and yes—I'm even looking for a co-founder to help us build what comes next. Thank you for being part of this journey. Whether you're a client who trusted us, a reader who engages with our work, or someone cheering from the sidelines—you've helped make this real. Here's to bringing science closer to society, one story at a time. — Suchitha
Scientific credibility today depends not only on the strength of your data but on how well your organization understands the environment that data enters. In biotech and medtech—where clinicians are overloaded, payers are cautious, and regulatory expectations are tightening—clarity comes from knowing the landscape your innovation must navigate. That’s where competitive intelligence (CI) becomes essential: not as surveillance, but as a structured, ethical lens on the realities shaping your field.
This edition explores what happens when CI and science storytelling work together. From grounding claims in context, to using domain-specific AI tools responsibly, to learning from companies that communicate with transparency, CI enables narratives that clinicians and regulators trust—not because they sound impressive, but because they reflect the world as it is.
As you read, consider this: CI isn’t about naming competitors—it’s about honouring your audience’s reality. When teams do that well, clarity becomes a strategic advantage.
Featured Insight
Why Market-Aware Narratives Build Stronger Trust
Scientific narratives resonate most when they acknowledge the landscape into which an innovation emerges. Clinicians, patients, reviewers, and partners evaluate new technologies against what they already use, understand, or distrust—making context a critical component of credibility.
A simple definition:
Competitive intelligence (CI) = publicly available insights about existing solutions, stakeholder needs, regulatory shifts, and scientific trends.
When CI informs science storytelling, teams communicate more:
Realistically — aligning with the standard-of-care and current evidence
Usefully — addressing the friction points stakeholders already experience
Transparently — clarifying where the innovation fits today
Credibly — avoiding hype and unsupported comparisons

Innovation Showcase
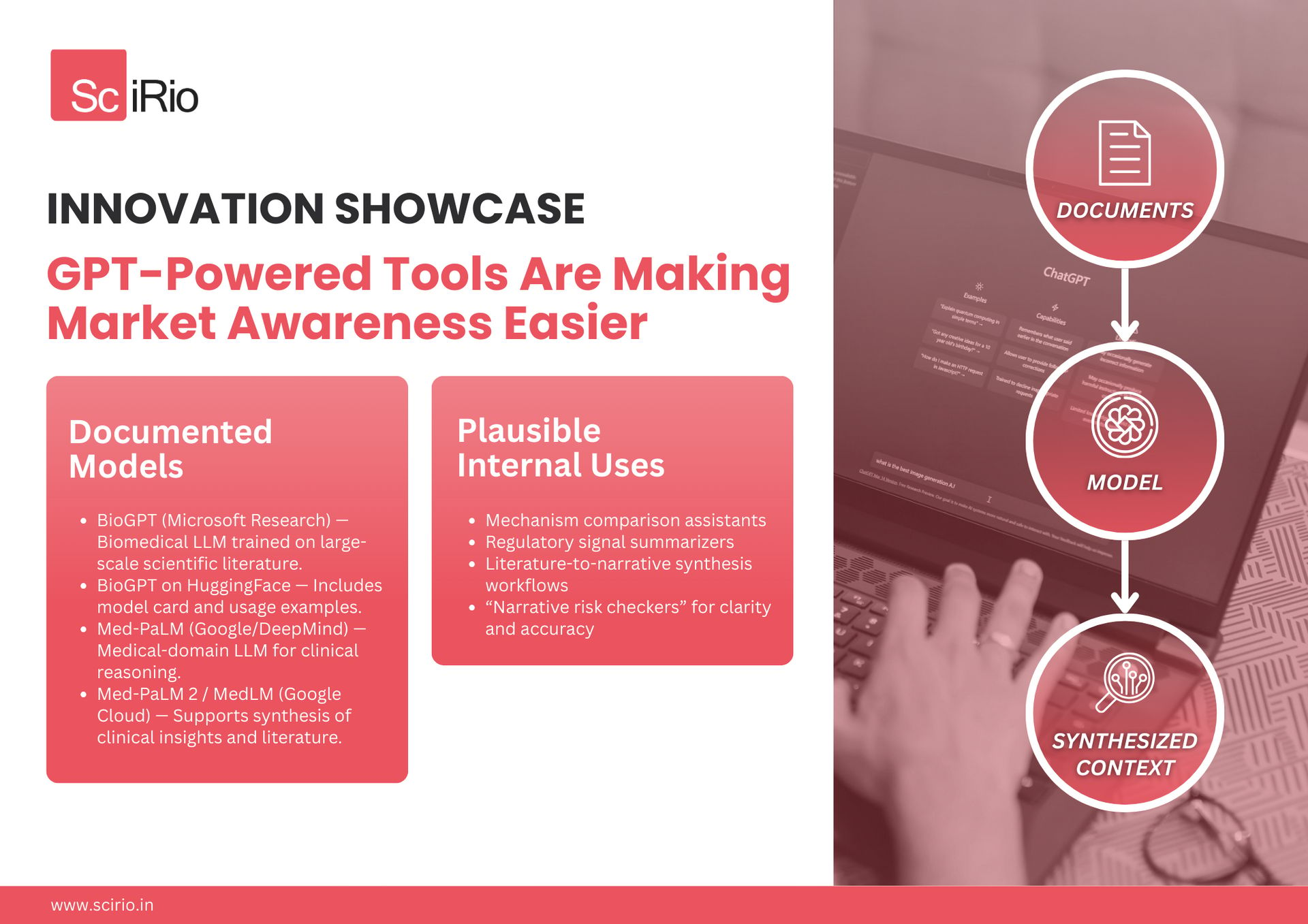
GPT-Powered Tools Are Making Market Awareness Easier
Domain-specific large language models (LLMs) have advanced rapidly, enabling better synthesis of biomedical information and clearer contextual insights.
Documented Models
BioGPT (Microsoft Research) — Biomedical LLM trained on large-scale scientific literature.
BioGPT on HuggingFace — Includes model card and usage examples.
Med-PaLM (Google / DeepMind) — Medical-domain LLM for clinical reasoning.
Med-PaLM 2 / MedLM (Google Cloud) — Supports synthesis of clinical insights and medical literature.
Speculative but Plausible Internal Uses
These are common internal applications—not commercial tools:
Mechanism comparison assistants
Regulatory signal summarizers
Literature-to-narrative synthesis workflows
“Narrative risk checkers” for clarity and accuracy
These tools help teams stay focused on evidence and context, rather than hype or intuition.
Practical Tools
Real CI Tools Readers Can Use Today
These public, ethical resources are widely used across biotech, medtech, and healthtech:
PubMed Clinical Queries — Rapid filtering of clinical studies and systematic reviews.
FDA 510(k) & De Novo Databases — Tracks medical device regulatory submissions; essential for medtech landscape context.
Drugs@FDA & Clinical Pharmacology Database — Track approvals and regulatory histories.
ClinicalTrials.gov — Identify trial volumes, endpoints, and active pipelines.
WHO Global Observatory on Health R&D — Highlights global research gaps.
CB Insights Health Tech Tracker — Macro-level healthtech and digital therapeutics trends.
Even non-CI professionals can use these tools to assess whether their narratives align with the real world.

From the Field
Market-Aware Storytelling in Action
1. Dexcom — Communicating Continuous Glucose Monitoring Within an Ecosystem
Dexcom describes a “growing ecosystem” of over 80 partners, acknowledging how continuous glucose monitoring fits into larger diabetes management workflows. G7 clearance materials emphasize accuracy and design improvements in the context of real-world use.
2. Guardant Health — Positioning Liquid Biopsy as Complementary
Guardant publicly frames liquid biopsy (Guardant360 CDx) as complementary to tissue testing through press releases.
The Guardant360 TissueNext launch reinforces that the test can be used “alone or in combination” with Guardant360 CDx.
These examples show that credibility grows when companies communicate with context rather than competition.

Behind the Scenes
How Teams Turn Market Insight Into a Clear, Ethical Science Story
Inside biotech and medtech organizations, CI-informed storytelling is a cross-functional collaboration. A simplified view of what happens:
1. CI Teams Surface the Signals
Publicly available insights: regulatory changes, trial activity, published data, and adoption patterns.
2. Medical Affairs Interprets the Clinical Meaning
They determine which insights matter for clinicians and patients.
3. Researchers Validate the Science
Bench and technical teams ensure statements represent the data accurately.
4. Regulatory Aligns What Can Be Said
Clarifying permissible claims, required qualifiers, and restricted statements.
5. Communications Builds the Narrative
Crafting clear, contextualized storytelling anchored in the real world.
Outcome
A narrative that is:
scientifically correct
contextualized and realistic
transparent about limitations
aligned with regulations
genuinely useful to stakeholders
This is how market insights become responsible science storytelling.

Community Corner
Question for You:
If your team doesn’t formally use CI, what do you rely on to understand the context your science fits into?
Share your thoughts with us on LinkedIn @SciRio.
Small Habit to Try:
Add a slide titled “Where This Innovation Fits” to every internal review.
This isn’t about naming competitors—just acknowledging the reality your audience already understands.
Missed our last edition? Read it here.
Final Word
You don’t need to be a CI specialist to create market-aware narratives. A few ethical tools, a disciplined approach to sourcing, and a commitment to transparency can elevate your storytelling—and ultimately deepen trust.
SciRio’s Blog
Imagine if the secrets of medicine and the mysteries of the cosmos were first passed down not in books or screens, but through songs and stories that filled ancient villages with curiosity. In part 1 of the blog series on Scicomm through the Ages, the author offers a sneak peek into how science communication has journeyed from charismatic healers and poetic verse to global exhibitions, television icons, and the digital age—always keeping our wonder alive. Discover how every era found creative ways to share scientific knowledge and connect people, and explore the full story on SciRio.